NICE (The National Institute For Health And Care Excellence)
OUR PROJECTS
The National Institute of Clinical Excellence (NICE) has recommended for the first treatment in two decades for sickle cell disease
The AOFAC Foundation are proud to have been involved. At AOFAC Foundation we believe that there could be a link between TTP and sickle cell disease and so also Lupus SLE and hope that our interest and participations will lead to research into any link between TTP, sickle cell disease and so also Lupus SLE.
NICE PRESS RELEASE
Hundreds of people will be eligible for a new treatment for sickle cell disease following draft guidance published today (5 October, 2020).
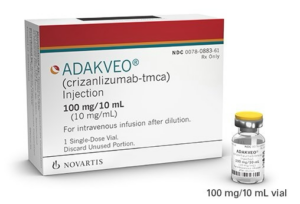
For the first time in 20 years, a new therapy for sickle cell disease is to be made available on the NHS. Crizanlizumab (Adakveo, Novartis) is recommended by NICE as a treatment option for preventing recurrent sickle cell crises in people aged 16 or over.
Here is the link for the full NICE draft guidance documents: https://www.nice.org.uk/guidance/indevelopment/gid-ta10470
Over 300 people a year will initially be eligible for the treatment via a Managed Access Agreement (MAA), increasing to more than 450 people in subsequent years.
Current treatments to prevent sickle cell crises include a tablet called hydroxycarbamide and regular blood transfusions, which are not always effective for people with severe disease. Crizanlizumab is a new intravenous treatment option that can be taken on its own or alongside hydroxycarbamide.
Clinical evidence suggests that people treated with crizanlizumab have significantly fewer sickle cell crises in a year than those receiving other standard treatment options.
However, there is high uncertainty about the long-term effectiveness of the treatment and the cost-effectiveness, so crizanlizumab could not be recommended for routine use on the NHS at this stage. Instead, the committee has recommended the treatment through the MAA with the NHS, which will allow people to access crizanlizumab while additional data is collected through clinical trials to address these uncertainties.
This decision was based on crizanlizumab being cost effective according to the terms of the MAA. The committee’s decision-making also took into account the high unmet need for treatments for sickle cell disease and NICE’s aim of reducing health inequalities.
ACHIEVEMENT
We are indeed very proud of us having a part in this new treatment for sickle cell disease but sad that it’s been 20 years since a new treatment drug for sickle cell is available and hoping it will be a treatment that can be made available for the benefit of all patients.

HEALTH CARE
